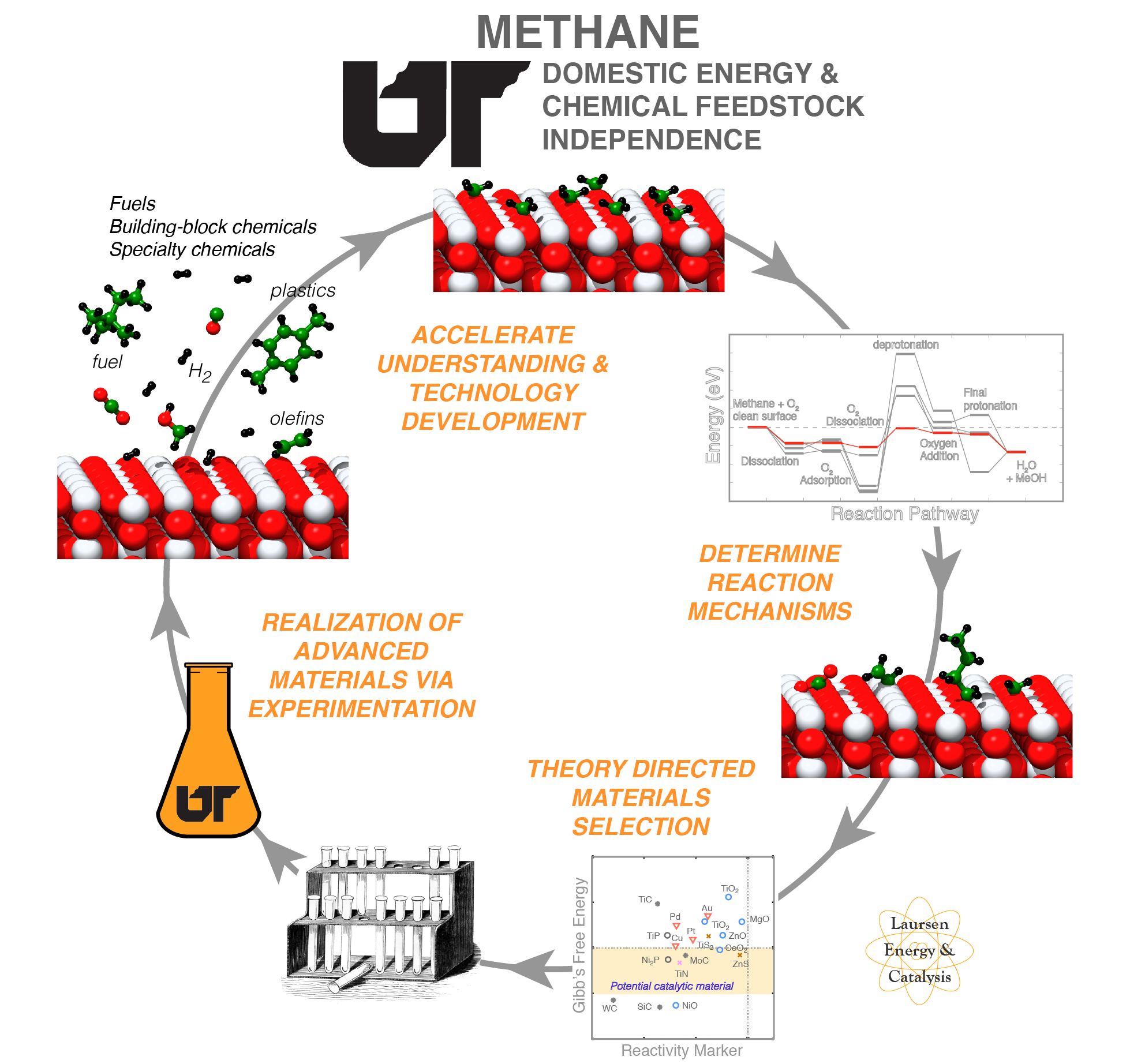
Noble metals are traditionally used as heterogeneous catalysts, but are costly and lack inherent tune-ability. Transition metal compounds - transition metals mixed with non-metals and semi-metals - show great promise as inexpensive, earth-abundant heterogeneous catalysts. These materials can present surface chemical reactivity similar to noble metals and present two distinctly tunable surface reaction sites - metal (cation) and semi/non-metal (anion). We focus on understanding how the surface chemical reactivity and material stability under reaction conditions are affected by utilizing various transition metals and semi/non-metal elements. Experimentally, these materials are synthesized and their overall catalytic performance is evaluated in-house. Our group is unique in that it employs a combination of quantum chemical modeling and experimental techniques, which has proven to greatly accelerate the discovery and optimization of advanced materials. The fundamental framework for catalyst design can be readily applied to a wide range of contemporary reactions. We are currently focused upon C-O, C-H, and C-C selective bond cleavage in the context of direct alkane functionalization and methane, CO2, and Biomass catalytic upgrading.
Noble metals are traditionally used as heterogeneous catalysts, but are costly and lack inherent tune-ability. Transition metal compounds - transition metals mixed with non-metals and semi-metals - show great promise as inexpensive, earth-abundant heterogeneous catalysts. These materials can present surface chemical reactivity similar to noble metals and present two distinctly tunable surface reaction sites - metal (cation) and semi/non-metal (anion). We focus on understanding how the surface chemical reactivity and material stability under reaction conditions are affected by utilizing various transition metals and semi/non-metal elements. Experimentally, these materials are synthesized and their overall catalytic performance is evaluated in-house. Our group is unique in that it employs a combination of quantum chemical modeling and experimental techniques, which has proven to greatly accelerate the discovery and optimization of advanced materials. The fundamental framework for catalyst design can be readily applied to a wide range of contemporary reactions. We are currently focused upon C-O, C-H, and C-C selective bond cleavage in the context of direct alkane functionalization and methane, CO2, and Biomass catalytic upgrading.
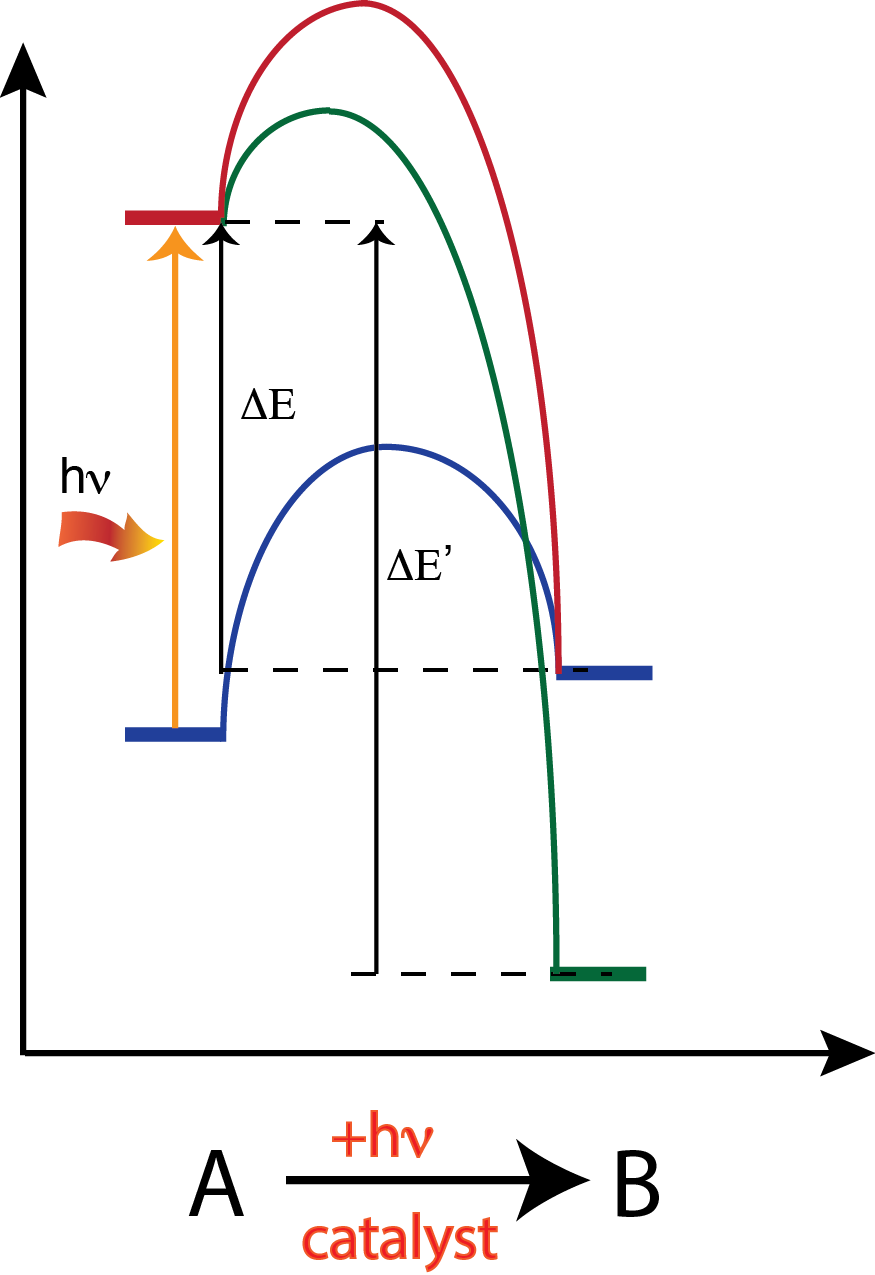
 Rising global energy demands, diminishing fossil fuel reserves, and growing concerns of global climate change have sparked great interest in the development of processes that transform CO2 into hydrocarbons and fuels. CO2 is a carbon resource that is readily available from the power generation industry and currently a waste product. Unfortunately, transforming CO2 into useful chemicals is difficult due to its thermochemical stability and general inertness. Conversion of CO2 commonly requires intense energy input or aggressive reducing agents. Our studies focus on designing highly efficient photocatalysts that reduce CO2 and H2O directly into value-added chemicals and fuels. Using exhaustive quantum chemical modeling we are able to investigate the energetics of surface reaction mechanisms and the stability of key reaction intermediates. The insights gained from theoretical calculations help us isolate potential photocatalysts, which are then synthesized and tested using well-defined experiments.
Rising global energy demands, diminishing fossil fuel reserves, and growing concerns of global climate change have sparked great interest in the development of processes that transform CO2 into hydrocarbons and fuels. CO2 is a carbon resource that is readily available from the power generation industry and currently a waste product. Unfortunately, transforming CO2 into useful chemicals is difficult due to its thermochemical stability and general inertness. Conversion of CO2 commonly requires intense energy input or aggressive reducing agents. Our studies focus on designing highly efficient photocatalysts that reduce CO2 and H2O directly into value-added chemicals and fuels. Using exhaustive quantum chemical modeling we are able to investigate the energetics of surface reaction mechanisms and the stability of key reaction intermediates. The insights gained from theoretical calculations help us isolate potential photocatalysts, which are then synthesized and tested using well-defined experiments.
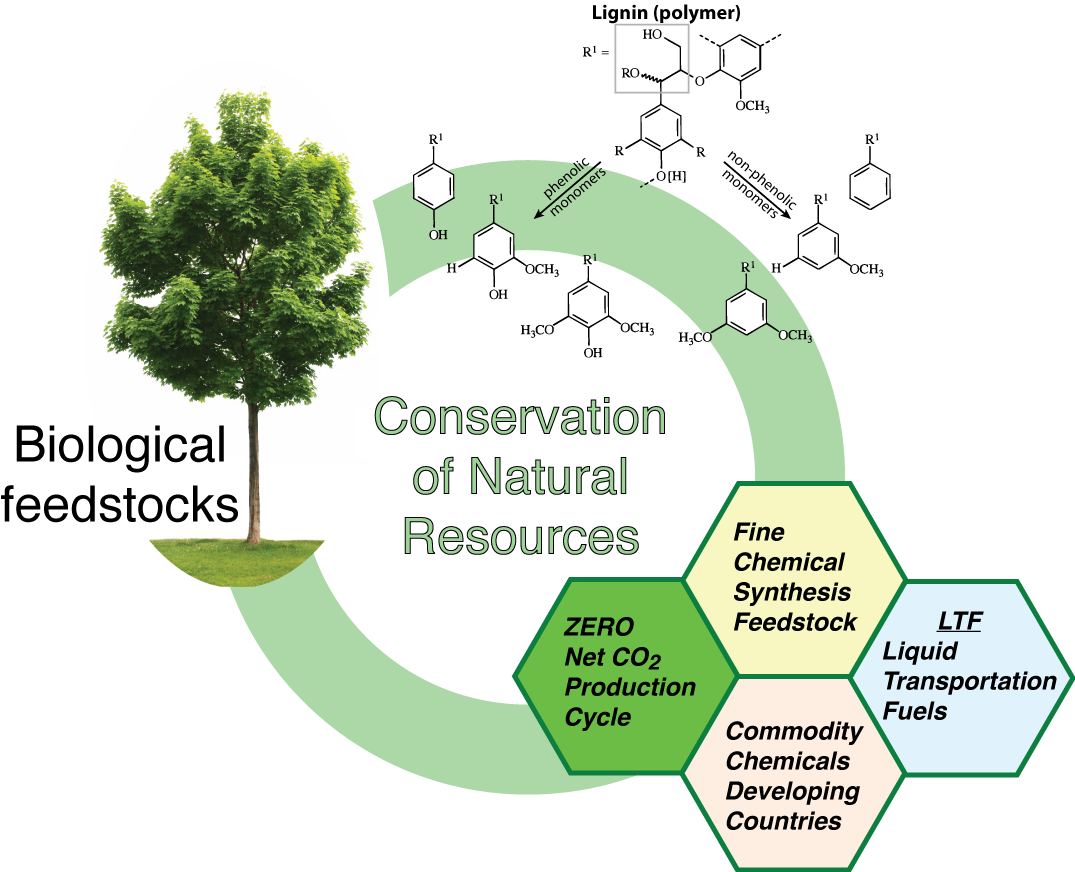
 Utilizing biomass as a source of fuel and chemicals has the potential to decrease domestic dependence upon foreign energy and hydrocarbon sources. It also promises to reduce the net production of CO2 by producing a CO2-neutral chemical feedstock. Our investigation focuses upon developing highly active and stable catalysts that effectively deoxygenate the organic fraction of pyrolysis oil. A combination of quantum chemical modeling and experimental techniques are applied to investigate the reaction mechanisms of biomass deoxygenation over transition metal oxide, carbide, nitride, sulfide, and phosphide solids. We have isolated the critical variables of interest in the design of these catalysts - the supply of reactive hydrogen, the ability of the surface to cleave C–O bonds, and the stability of elemental carbon in connection to coke formation.
Utilizing biomass as a source of fuel and chemicals has the potential to decrease domestic dependence upon foreign energy and hydrocarbon sources. It also promises to reduce the net production of CO2 by producing a CO2-neutral chemical feedstock. Our investigation focuses upon developing highly active and stable catalysts that effectively deoxygenate the organic fraction of pyrolysis oil. A combination of quantum chemical modeling and experimental techniques are applied to investigate the reaction mechanisms of biomass deoxygenation over transition metal oxide, carbide, nitride, sulfide, and phosphide solids. We have isolated the critical variables of interest in the design of these catalysts - the supply of reactive hydrogen, the ability of the surface to cleave C–O bonds, and the stability of elemental carbon in connection to coke formation.